Mississippi has seen a dramatic slowdown in its sprint to COVID-19 herd immunity. Residents of the Magnolia State have been getting vaccinated at a rapidly decreasing rate, with each consecutive week in the month of April showing double-digit declines in the percentage of Mississippians getting their first or second shots.
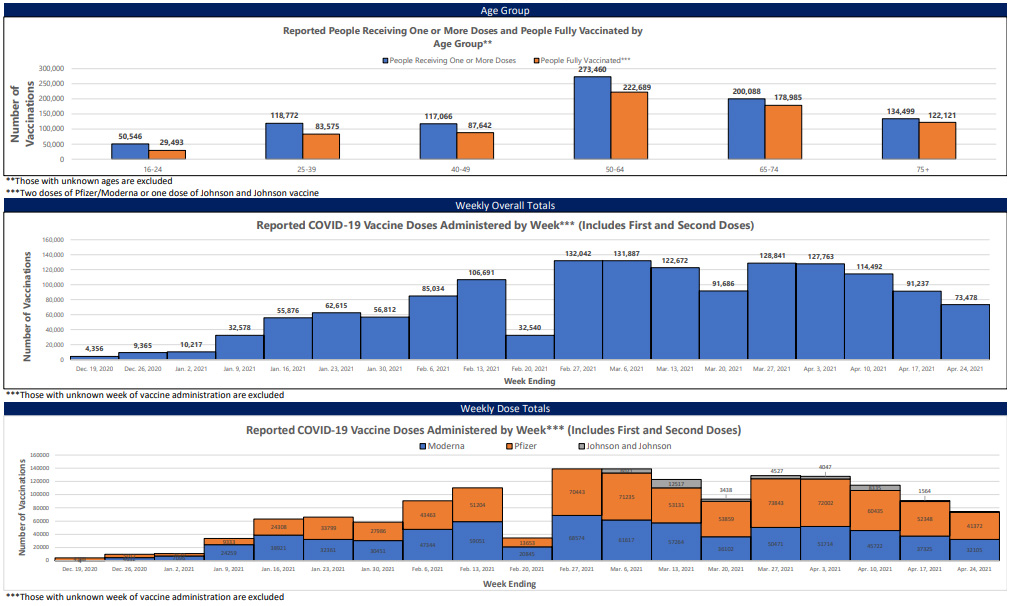
Between Feb. 27 and April 3, weekly reports held steady at roughly 130,000 new vaccinations each week, with only a temporary blip between March 13 and 20, due to weather closures of Mississippi State Department of Health vaccination sites.
Between April 3 and April 24, however, new vaccinations cratered from 127,763 to 73,478, a 43% decrease over the course of a single month. While this period does include the nationwide pause on use of the Johnson & Johnson vaccine, this does not account for the rapid decrease in new shots. MSDH data show that Johnson & Johnson shots have consistently represented only a small fraction of the state’s vaccinations. New Pfizer and Moderna vaccinations have shown the same precipitous decline over April.
Downturn Now A National Trend
The consistent decline in new vaccinations is more pronounced in Mississippi than it is elsewhere, but a downward trend is now visible on the national level.
The Washington Post reported last week that “[a]bout 3 million Americans are getting vaccinated daily, an 11 percent decrease in the seven-day average of daily shots administered over the past week.”
The decline in new vaccinations lines up with the April 13 pause on Johnson & Johnson, which the Centers for Disease Control and Food and Drug Administration lifted earlier this week. This has led both political and medical leadership to express concerns that the extremely rare side-effects of the Johnson & Johnson vaccine seen in some U.S. women are driving vaccine hesitancy across the country.
The slowdown in vaccinations comes at a crucial moment in the U.S. campaign for herd immunity. The Washington Post reports that “half of all eligible Americans have received at least one vaccine dose.”
In Mississippi, that number is definitively lower, with 936,832 Mississippians having received at least one dose of any COVID-19 vaccine. With roughly 705,000 Mississippians under age 18, roughly 40% of the state’s eligible population has received at least one shot. That number goes down somewhat if the state’s 16- and 17-year-olds are included, as they already qualify for the Pfizer vaccine.
Johnson & Johnson Still Paused in Mississippi
While both the CDC and the FDA support a return to the use of the J&J vaccine, MSDH has yet to formally recommit to use of the vaccine.

MSDH Communications Director Liz Sharlot sent the Mississippi Free Press a statement this afternoon confirming that the temporary pause was still in effect.
“As of now, the Mississippi State Department of Health continues to pause Johnson and Johnson vaccine. The Agency will review additional information and will advise the media and public if and when Mississippi resumes administration of the vaccine,” Sharlot wrote.
A decision on the J&J vaccine is expected to come later this week.
Data: Risk of Blood Clots Extremely Small
A University of Oxford study confirmed what many public-health experts have said since the first reports of severe blood clots associated with the AstraZeneca vaccine emerged in Europe: the risk of these same dangerous clots is far more significant for those infected with COVID-19 than in any vaccine studied.
Researchers found that, out of 513,284 COVID-19 patients examined for the study, 20 developed cerebral venous thrombosis, the extremely dangerous blood clots found in 6 U.S. women who had previously received the Johnson & Johnson vaccine—out of 6.8 million total shots in the country.
The study’s authors explained to CBS News in an April 19 interview that the data show clots to be vanishingly rare in all cases, but that COVID-19 itself presents a significantly greater chance of causing CVSTs than any vaccine the Oxford study considered.
The 10-times-greater chance of developing CVSTs accompanies the vast collection of other serious organ damage researchers associate with COVID-19, even in young and healthy patients.
In addition to recommending a return to the use of the J&J vaccine, CDC Director Rochelle Walensky confirmed last week that pregnant women should get the COVID-19 vaccine, following the results of a study that showed no heightened safety risks for pregnant women or babies from the vaccines.










